Answer:
21 mL of NaOH is required.
Step-by-step explanation:
Balanced reaction:

Number of moles of HBr in 11.0 mL of 0.30 M HBr solution
=
 moles = 0.0033 moles
moles = 0.0033 moles
Let's say V mL of 0.16 M NaOH solution is required to reach equivalence point.
So, number of moles of NaOH in V mL of 0.16 M NaOH solution
=
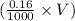 moles = 0.00016V moles
moles = 0.00016V moles
According to balanced equation-
1 mol of HBr is neutralized by 1 mol of NaOH
So, 0.0033 moles of HBr are neutralized by 0.0033 moles of NaOH
Hence,
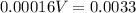
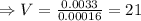
So, 21 mL of NaOH is required.