Answer: HA has lowest pH and it is the most acidic as compared to the rest of given acids.
Step-by-step explanation:
We know that relation between
 and
and
 is as follows.
is as follows.
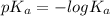
This means that more is the value of
 , smaller will be the
, smaller will be the
 . Also, more is the value of
. Also, more is the value of
 smaller will be the pH of a solution.
smaller will be the pH of a solution.
As, larger is the value of
 more negative will be the
more negative will be the
 value. Hence, stronger will be the acid.
value. Hence, stronger will be the acid.
In the given options, HA has the smallest
 value.
value.
Therefore, we can conclude that HA has lowest pH and it is the most acidic as compared to the rest of given acids.