Answer:
A.
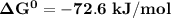 ; as such the reaction is said to be spontaneous since the value of
; as such the reaction is said to be spontaneous since the value of
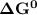 is negative.
is negative.
B.
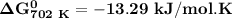 and the reaction is spontaneous
and the reaction is spontaneous
Step-by-step explanation:
The equation for this chemical reaction is :
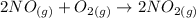
Using the following relation to calculate
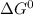 ;
;
![\Delta G^0 = [2(\Delta G^0_{NO_(2(g))}] - [1(\Delta G^0_{O_(2(g))})+ 2(\Delta G^0_{NO_(g)})]](https://img.qammunity.org/2021/formulas/chemistry/college/s3j38eg1ct8fl806f8s9ztq7nryykv5bz0.png)
At 298 K; the standard Gibbs Free Energy for the formation are as follows:
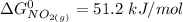
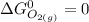
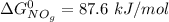
Replacing them into the above equation;
![\Delta G^0 = [2(51.2 \ kJ/mol}] - [1(0)+ 2(87.6 \ kJ/mol})]](https://img.qammunity.org/2021/formulas/chemistry/college/b5wyxef6dhxgi6xjc2lwupucmxkji06o1x.png)
![\Delta G^0 = [102.4 \ kJ/mol}] - [175.2 \ kJ/mol})]](https://img.qammunity.org/2021/formulas/chemistry/college/cdndbs7iudf5feiuzl19mhwdb37zj7khlb.png)
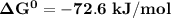
Thus;
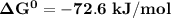 ; as such the reaction is said to be spontaneous since the value of
; as such the reaction is said to be spontaneous since the value of
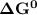 is negative.
is negative.
B.
Using the same above chemical equation;
The relation used for calculating
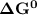 of the reaction when the temperature is 702 K is:
of the reaction when the temperature is 702 K is:
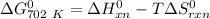
where;
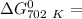 Gibbs free energy of the reaction at 702 K
Gibbs free energy of the reaction at 702 K
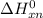 = standard enthalpy of the reaction = -116.2 kJ/mol
= standard enthalpy of the reaction = -116.2 kJ/mol
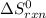 = standard entropy of the reaction = -146.6 J/mol/K
= standard entropy of the reaction = -146.6 J/mol/K
Temperature T = 702 K
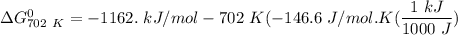
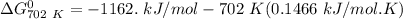
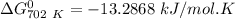
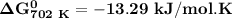
Thus
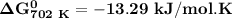 and the reaction is spontaneous
and the reaction is spontaneous