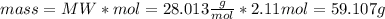Answer:
H2 consumed 4.22 mol
N2 produced 59.107 g
Step-by-step explanation:
Balanced equation:
2NO (g) + 2H2 (g) N2 (g) + 2H2O (l)
- To perform the calculations, the molecular weights of the following compounds must be known:
H2O MW = 18.02 g/mol
N2 MW = 28.01 g/mol
To determine the moles of H2O produced, the following formula should be used:
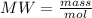
The value of moles is cleared:
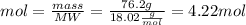
- Now, to calculate the grams of N2 consumed, we look at the balanced equation and note that 2 moles of H2 produce 1 mole of N2. Therefore, through said observation, the amount of moles of H2 consumed can be determined.
2 mol H2 ⇒ 1 mol N2
4.22 mol H2 ⇒ X
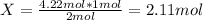
To calculate the mass of H2 consumed, the molecular weight equation is used again: