Answer:
a)
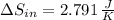 , b)
, b)
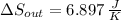 , c)
, c)
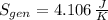 , d) Due to irreversibilities due to temperature differences.
, d) Due to irreversibilities due to temperature differences.
Step-by-step explanation:
a) The change in entropy of the hot reservoir is:
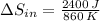
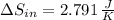
b) The change in entropy of the cold reservoir is:
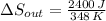
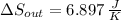
c) The total change in entropy of the Universe is modelled after the Second Law of Thermodynamics. Let assume that process is steady:
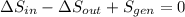
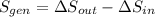
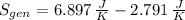
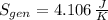
d) Since irreversibilities create entropy as process goes by. The main source of irreversibilities is the existence of temperature differences.