Answer:
Approximately
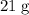 .
.
Step-by-step explanation:
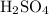 (a diprotic acid) reacts with
(a diprotic acid) reacts with
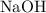 (a monoprotic base) at a one-to-two ratio:
(a monoprotic base) at a one-to-two ratio:
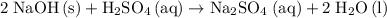 .
.
In other words, if
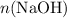 and
and
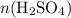 represent the number of moles of the two compounds reacted, then:
represent the number of moles of the two compounds reacted, then:
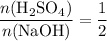 .
.
Look up the relative atomic mass data on a modern periodic table:
Calculate the (molar) formula mass of
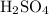 and
and
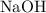 :
:
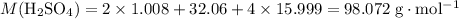 .
.
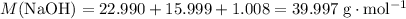 .
.
Calculate the number of moles of formula units in that
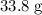 of
of
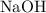 :
:
 .
.
Apply the ratio
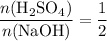 to find the (maximum) number of moles of
to find the (maximum) number of moles of
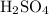 that would react with the
that would react with the
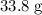 of
of
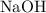 :
:
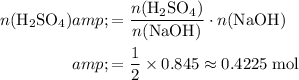 .
.
Calculate the mass of that
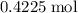 of
of
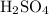 :
:
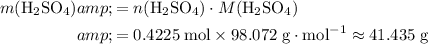 .
.
When the maximum amount of
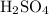 is reacted, the minimum would be in excess. Hence, the minimum mass of
is reacted, the minimum would be in excess. Hence, the minimum mass of
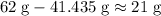 (rounded to two significant figures.)
(rounded to two significant figures.)