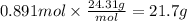Answer:
21.7 g
Step-by-step explanation:
Step 1: Write the balanced equation
3 Mg + N₂ → Mg₃N₂
Step 2: Calculate the moles corresponding to 8.33 g of nitrogen
The molar mass of N₂ is 28.01 g/mol.
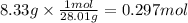
Step 3: Calculate the moles of magnesium that reacts with 0.297 moles of nitrogen
The molar ratio of Mg to N₂ is 3:1. The reacting moles of Mg are 3/1 × 0.297 mol = 0.891 mol
Step 4: Calculate the mass corresponding to 0.891 moles of magnesium
The molar mass of Mg is 24.31 g/mol.