Answer:
0.031 m
HgO(s) ⇒ Hg(l) + 1/2 O₂(g)
Chemical change
Element
Step-by-step explanation:
A 75 gram solid cube of mercury (II) oxide has a density of 2.4 × 10³ kg/m³. What is the length of one side of the cube in cm?
Step 1: Convert the mass to kilograms
We will use the relationship 1 kg = 1,000 g.
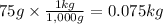
Step 2: Calculate the volume (V) of the cube
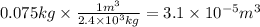
Step 3: Calculate the length (l) of one side of the cube
We will use the following expression.
![V = l^(3) \\l = \sqrt[3]{V} = \sqrt[3]{3.1 * 10^(-5) m^(3) }=0.031m](https://img.qammunity.org/2021/formulas/chemistry/college/8keeg7gv43810jfm8o5t3e7lcahc2d61re.png)
The mercury (II) oxide completely dissociates and forms liquid mercury and oxygen gas. Write a balanced chemical equation and indicate if this process is a chemical or physical change?
The balanced chemical equation is:
HgO(s) ⇒ Hg(l) + 1/2 O₂(g)
This is a chemical change because new substances are formed.
The oxygen gas escapes and now you are left with liquid grey substance. Is this grey substance a compound, element, homogeneous mixture or heterogeneous mixture?
The liquid gray substance is Hg(l), which is an element because it is formed by just one kind of atoms.