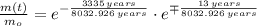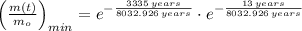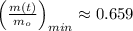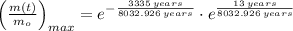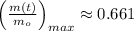Answer:
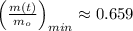 and
and
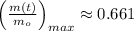
Explanation:
The equation of the isotope decay is:
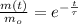
14-Carbon has a half-life of 5568 years, the time constant of the isotope is:
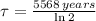
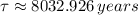
The decay time is:
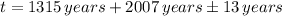 (There is no a year 0 in chronology).
(There is no a year 0 in chronology).
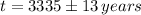
Lastly, the relative amount is estimated by direct substitution: