Answer:
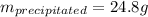
Step-by-step explanation:
Hello,
In this case, since at 60 °C, 108 grams of ammonium bromide are completely dissolved in 100 grams of water for a saturated solution, once it is cooled to 30 °C, wherein only 83.2 grams are completely dissolved in 100 grams of water, the following mass will precipitate:
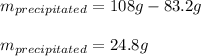
Best regards.