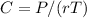Answer:
Step-by-step explanation:
From the question, osmotic pressure exerted by a solution is equal to the MOLARITY multiplied by the absolute TEMPERATURE and the GAS CONSTANT r.
Let P = osmotic pressure,
C = molarity, then
T = absolute temperature
r=gas constant
The Osmotic pressure Equation exerted by a solution
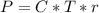
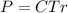
Then it was required in the question to write an equation that will let you calculate the molarity c of this solution, and this equation should contain ONLY symbols
C= molarity of the solution
P=osmotic pressure
r = gas constant
T= absolute temperature
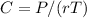
The equation that will let us calculate the molarity c of this solution =