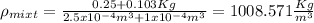Answer:
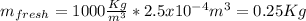
And we can do a similar procedure for the sea water:
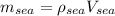
And after convert the volume to m^3 we got:
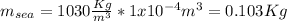
And then the density for the mixture would be given by:
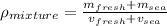
And replacing we got:
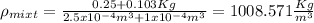
Explanation:
For this case we can begin calculating the mass for each type of water:
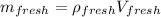
And after convert the volume to m^3 we got:
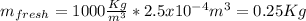
And we can do a similar procedure for the sea water:
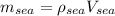
And after convert the volume to m^3 we got:
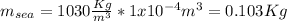
And then the density for the mixture would be given by:
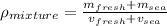
And replacing we got: