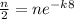Answer:
k = 0.086
Explanation:
We have the equation
y=ne^(-kt)
where y is the amount of radioactive material after t hours, n is the initial amount of radioactive material, and k is some constant.
We know the following data:
- y = n/2 (half of a radioactive element remains)
Replacing into the equation and solving for k: