Answer:
The entropy change of the Universe that occurs is 19.346 J/K
Step-by-step explanation:
Given;
temperature of the sun,
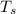 = 5,300 K
= 5,300 K
temperature of the Earth,
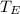 = 293 K
= 293 K
radiation energy transferred by the sun to the earth, E = 6000 J
The sun loses Q of heat and therefore decreases its entropy by the amount
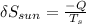
The earth gains Q of heat and therefore increases its entropy by the amount
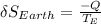
The total entropy change is:

Therefore, the entropy change of the Universe that occurs is 19.346 J/K