Answer:
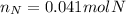
Step-by-step explanation:
Hello,
In this case, for this mole-mass relationship, we are able to compute the moles of nitrogen atoms by firstly obtaining the moles of the given compound, considering its molar mass that is 194 g/mol:
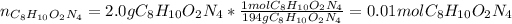
Then, by knowing that one mole of the given compound has four moles of nitrogen atoms, we apply the following relationship:
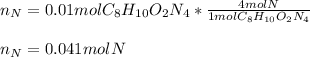
Best regards.