Answer:
Wavelength,
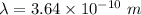
Step-by-step explanation:
We have,
Velocity an electron is
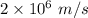
Mass of an electron is
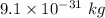
It is required to find the wavelength of electron. The De-Broglie wavelength of an electron is given by :
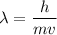
h is Planck's constant
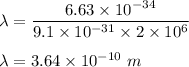
So, the wavelength of an electron is
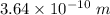 .
.