Answer:
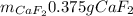
Step-by-step explanation:
Hello,
In this case, for the studied reaction:
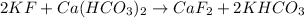
Thus, the first step is to compute the reacting moles of potassium fluoride by using its volume and molarity:
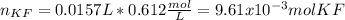
Then, we apply the 2:1 molar ratio between potassium fluoride and calcium fluoride to compute the produced moles of calcium fluoride:
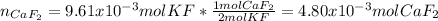
Finally, by using the molar mass of calcium fluoride (78.07 g/mol) we can compute its produced grams:
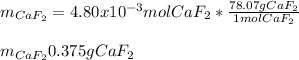
Best regards.