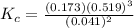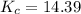Complete Question
The complete question is shown on the first uploaded image
Answer:
The concentration equilibrium constant is
Step-by-step explanation:
The chemical equation for this decomposition of ammonia is
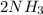 ↔
↔
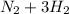
The initial concentration of ammonia is mathematically represented a
![[NH_3] = (n_1)/(V_1) = (29)/(75)](https://img.qammunity.org/2021/formulas/chemistry/college/mwcskd86v7obivf7wp21p1qvtrdwzjcv59.png)
![[NH_3] = 0.387 \ M](https://img.qammunity.org/2021/formulas/chemistry/college/doja3usbz9gb476xt918p6y6v4iya0ca46.png)
The initial concentration of nitrogen gas is mathematically represented a
![[N_2] = (n_2)/(V_2)](https://img.qammunity.org/2021/formulas/chemistry/college/ir5v6s8exph7v3ca04om91z2yo5d8gvyqr.png)
![[N_2] = 0.173 \ M](https://img.qammunity.org/2021/formulas/chemistry/college/mco0whxxhfkc996uzzrlbijmmsad3yt7k6.png)
So looking at the equation
Initially (Before reaction)
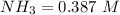

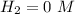
During reaction(this is gotten from the reaction equation )
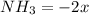 (this implies that it losses two moles of concentration )
(this implies that it losses two moles of concentration )
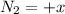 (this implies that it gains 1 moles)
(this implies that it gains 1 moles)
 (this implies that it gains 3 moles)
(this implies that it gains 3 moles)
Note : x denotes concentration
At equilibrium
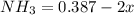
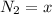
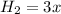
Now since
![[NH_3] = 0.387 \ M](https://img.qammunity.org/2021/formulas/chemistry/college/doja3usbz9gb476xt918p6y6v4iya0ca46.png)
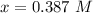
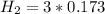
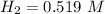
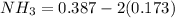
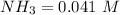
Now the equilibrium constant is
![K_c = ([N_2][H_2]^3)/([NH_3]^2)](https://img.qammunity.org/2021/formulas/chemistry/college/ero2ee1ox4s9t8d47hc0ifbhvdywsvcesz.png)
substituting values