Answer:
0.202M
Step-by-step explanation:
you will want to use C₁V₁=C₂V₂ to solve for this.
C₁ is the initial concentration, in this problem it is .458 mol/L or M
V₁ is the initial volume, so it will be the 22.0 mL or 0.0220L
C₂ is the new concentration but here it is unknown.
V₂ is the new volume which is 50.0 mL or 0.0500L.
1. So now we plug this information in to the equation:
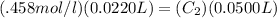
2. We then do algebra to get C₂ alone:
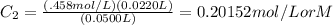
The liters cancel out and take note of the significant figures, there are 3 digits in all numbers in the question, so that means your answer must contain 3 sig figs. The answer 0.0202 rounded.
** instead of converting the mL to L you can also just keep them mL since they will be crossed out anyway. You will still get the correct answer.
Hope this help you! good luck :)