Answer:
Solubility cannot be calculated.
Step-by-step explanation:
To calculate the solubility of X it is necessary to know the value of the mass of the solute (X) that can be dissolved in 100 g of water.
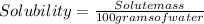
Taking into account that we do not know the value of the mass of the solution, therefore the value of the solubility of the compound cannot be determined.