Answer: 1. There are
 molecules of sulfuric acid in the solution.
molecules of sulfuric acid in the solution.
2. 0.093 moles of baking soda have been used.
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number
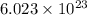 of particles.
of particles.
To calculate the moles, we use the equation:
1.
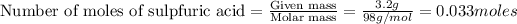
1 mole of
 contains =
contains =
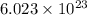 molecules of sulfuric acid
molecules of sulfuric acid
Thus mole of
 contains =
contains =
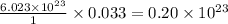 molecules of sulfuric acid
molecules of sulfuric acid
There are
 molecules of sulfuric acid in the solution.
molecules of sulfuric acid in the solution.
2.
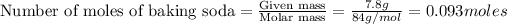
0.093 moles of baking soda have been used.