Answer: The solubility of X in water is
 g/ml.
g/ml.
Step-by-step explanation:
The given data is as follows.
Volume of sample water = 46 ml
Temperature =
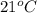
After vaporization, washes and then drying the weight of mineral X = 0.87 g
This means that 46.0 ml of water contains 0.87 g of X. Therefore, grams present in 1 ml of water will be calculated as follows.
1 ml of water =
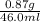
=
 g/ml
g/ml
Therefore, we can conclude that solubility of X in water is
 g/ml.
g/ml.