Answer:
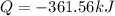
Step-by-step explanation:
Hello,
In this case, the decomposition of hydrogen peroxide is given by:
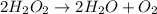
Which occurs in gaseous phase, therefore the enthalpy of reaction is:
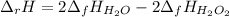
Oxygen is not included as it is a pure element. The enthalpies of formation for both hydrogen peroxide and water are -136.11 and -241.83 kJ/mol respectively, so we compute the enthalpy of reaction:
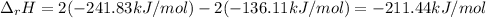
Then, the total heat that is released for 1.71 mol of hydrogen peroxide is:
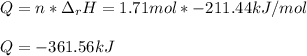
Whose sign means a released heat.
Regards.