Answer:
-
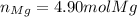
-
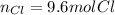
-
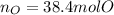
Step-by-step explanation:
Hello,
In this case, for the given 4.90 moles of magnesium perchlorate, we can compute the moles of each atom by identifying the subscript each atom has in the molecule as shown below:
- Moles of magnesium atoms: here, one mole of magnesium perchlorate has only one mole of magnesium atom (subscript is one), this the moles of magnesium atoms are also 4.90 moles.
- Moles of chlorine atoms: here, one mole of magnesium perchlorate has two moles of chlorine atoms as it has a two out of the parenthesis enclosing the perchlorate anion, thus, we have:
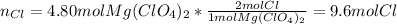
- Moles of oxygen atoms: here, one mole of magnesium perchlorate has eight moles of oxygen atoms as it has a four in the oxygen subscript and a two out of the parenthesis enclosing the perchlorate anion, thus, we have:
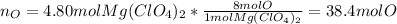
Best regards.