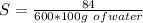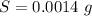Answer:
The solubility is
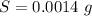
Step-by-step explanation:
From the question we are told that
The volume of the solution is
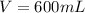
The initial temperature is
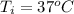
The final temperature is
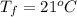
The additional precipitate is
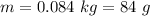
Yes because the solubility of the substance X is the amount of X needed to saturate a unit volume of the solvent (for solubility of a solute to be calculated the solute must be able to saturate the solvent)
now we see that the substance X saturated the solvent because a precipitate was formed which the student threw away
The solubility at 21 ° C is mathematically represented as
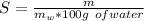
Mass of water(
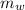 ) in the solution is mathematically represented as
) in the solution is mathematically represented as
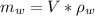
Where
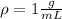
So
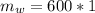
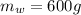
So