Answer:
1.1 mol of O2
Step-by-step explanation:
First we need the balance chemical equation which is
2 H2O2 -------> 2 H2O + O2
This is important because in stoichiometry you can go from units of one thing to other by using mole ratios, here the mole ratio is 2 mol of H2O2 for one mole of O2.
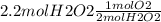 = 1.1 mol of O2
= 1.1 mol of O2