Answer:
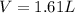
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
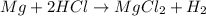
Next, we compute the reacting moles of each reactants:
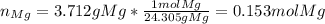
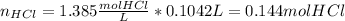
Then, as magnesium and hydrohloric acid are in a 1:2 molar ratio 0.153 moles of magnesium will completely react with 0.306 moles of hydrochloric acid yet we only have 0.144 moles, therefore, limiting reactant is hydrochloric acid. Thus, we compute the produced moles of hydrogen:
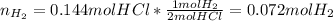
Finally, we use the ideal gas equation with T=298K and 1atm (STP conditions) to compute the liters of hydrogen gas:
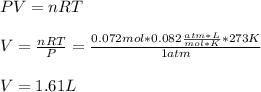
Best regards.