Answer:
a. the system is at equilibrium.
Step-by-step explanation:
Hello,
In this case, the undergoing chemical reaction is:
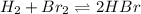
Thus, the law of mass action is given by:
![Keq=([HBr]^2)/([H_2][Br_2]) =57.6](https://img.qammunity.org/2021/formulas/chemistry/college/ny0jkkdwu4nx7adoaq0bto32x2x5kvvoli.png)
Nonetheless, for the given point of 4.67 × 10^-3M bromine gas, 2.14 × 10^−3 hydrogen gas, and 2.40 × 10^−2M hydrogen bromide gas we should compute the reaction quotient in order to know whether the direction of the reaction is to left or to right, thus:
![Q=([HBr]^2)/([H_2][Br_2]) =((2.40x10^(-2))^2)/((4.67x10^(-3))(2.14x10^(-3))) \\\\Q=57.6](https://img.qammunity.org/2021/formulas/chemistry/college/qp3sk88ag0a1e67wd72wuuyolzdt4f2riy.png)
Therefore, since Keq=Q, we say that the system is at equilibrium, for that reason, the answer is a.
Best regards.