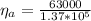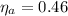Answer:
The power output of this engine is
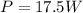
The the maximum (Carnot) efficiency is
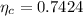
The actual efficiency of this engine is
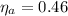
Step-by-step explanation:
From the question we are told that
The temperature of the hot reservoir is
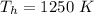
The temperature of the cold reservoir is
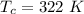
The energy absorbed from the hot reservoir is
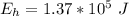
The energy exhausts into cold reservoir is
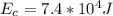
The power output is mathematically represented as
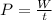
Where t is the time taken which we will assume to be 1 hour = 3600 s
W is the workdone which is mathematically represented as
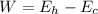
substituting values
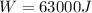
So
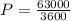
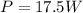
The Carnot efficiency is mathematically represented as
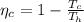
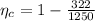
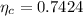
The actual efficiency is mathematically represented as
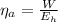
substituting values