Answer:
The new volume is 65.1 mL.
Step-by-step explanation:
We have,
Initial volume of the gas is 100 mL
Initial temperature is 300 degrees Celsius
It is required to find the volume the gas will occupy at 100 degrees Celsius. Charles law gives the relation between volume and temperature such that,
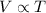
So,
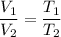
V₂ is new volume
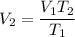
V₁ = 100 mL
T₁ = 300 C = 573.15 K
T₂ = 100 C = 373.15 K
Plugging into formula we get :

So, the new volume is 65.1 mL.