Answer:
The new temperature is 527.15 ºC.
Step-by-step explanation:
Charles’s law states that the volume of a fixed amount of gas maintained at constant pressure is directly proportional to the absolute temperature of the gas (the absolute temperature is the Kelvin temperature).
We need to calculate the temperature after the expansion, that is T₂. For that, we use Charles' law:
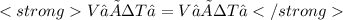
Because we have to use the absolute temperature, we convert ºC to K adding 273.15:
T₁ = 127 ºC + 273.15 ºC = 400.15 K
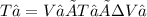
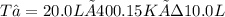
T₂ = 800.3 K
We substract 273.15 to the result to convert it back to ºC:
T₂ = 800.3 - 273.15 = 527.15 ºC.