Answer:
2.4 × 10⁻⁴ M/s
Step-by-step explanation:
Step 1: Given data
- Initial concentration of A ([A]₀): 0.054 M
- Concentration of A after a certain time ([A]): 0.032 M
Step 2: Convert the time to seconds
We will use the relationship 1 min = 60 s.
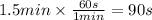
Step 3: Calculate the rate of the reaction
Since A is a reactant, we will calculate the rate of the reaction (r) using the following expression.
![r=-(\Delta [A])/(t) =-([A]-[A]_0)/(t) =-(0.032M-0.054M)/(90s) =2.4 * 10^(-4) M/s](https://img.qammunity.org/2021/formulas/chemistry/college/kqxyuegtjn15whaktmti46z3w69dga3uwp.png)