Answer:
The wavelength of the monochromatic light is 486.2 nm.
Step-by-step explanation:
The illumination of the hydrogen atom by the monochromatic light causes an absorption of energy by its electrons which causes an excitation. After a period, the particle de-excites (decays) losing the absorbed energy and falls back to its initial state releasing the energy in the form of a photon. This photon can be observed as a colored light of the Balmer series.
From Rydberg's expression,
1/λ=−R(
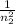 −
−
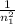 )
)
The transition of the electron is from n = 2 to 4, so that;
1/λ = R (
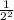 -
-
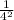 )
)
= 1.097 x
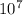 (
(
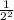 -
-
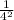 )
)
1/λ = 2056875
So that,
λ =
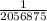
= 4.8617 x
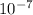 m
m
The wavelength of the monochromatic light is 486.2 nm.