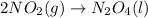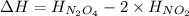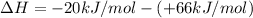Answer:
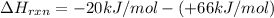
Step-by-step explanation:
Heat of reaction or enthalpy change is the energy released or absorbed during the course of the reaction.
It is calculated by subtracting the enthalpy of reactants from the enthalpy of products.
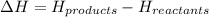
 = enthalpy change = ?
= enthalpy change = ?
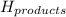 = enthalpy of products
= enthalpy of products
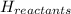 = enthalpy of reactants
= enthalpy of reactants
For the given reaction :