Answer:
The concentration of the solution is 5.8168 ×
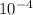 mol.
mol.
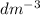
Step-by-step explanation:
Here, we want to calculate the concentration of the solution.
The unit of this is mol/dm^3
So the first thing to do here is to calculate the number of moles of the solute present, which is the number of moles of AlCO3
The number of moles = mass/molar mass
molar mass of AlCO3 = 27 + 12 + 3(16) = 27 + 12 + 48 = 87g/mol
Number of moles = 33.4/87 = 0.384 moles
This 0.384 moles is present in 660 L
x moles will be present in 1 dm^3
Recall 1 dm^3 = 1L
x * 660 = 0.384 * 1
x = 0.384/660 = 0.00058168 = 5.8168 * 10^-4 mol/dm^3