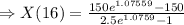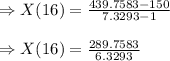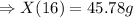Answer: 45. 78 g is formed in 16 minutes.
Step-by-step explanation:
Let
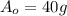 and
and
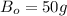
we know that
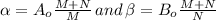
According to the question to create
 part of the chemical C we will need 2 part of A and one part of B.
part of the chemical C we will need 2 part of A and one part of B.
Therefore, M = 2 and N = 1.
Now, we can easily solve for the value of α and β.
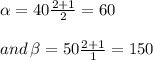
Now, the differential equation must be:
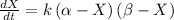
I separate the variable and solve the equation
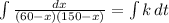
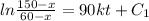
By using
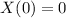 ,
,
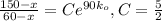
and using
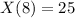 and solving for the value of 'k'
and solving for the value of 'k'
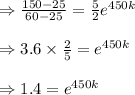
Taking 'ln' both side, we get
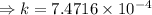
We obtain:
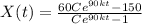
Now, for the 16 min