Answer:
Increases by a factor of 8.5
Step-by-step explanation:
Hello,
In this case, with the given data, we can apply the Boyle's law in order to understand the pressure-volume behavior as an inversely proportional relationship:

Thus, for the given pressure decrease we have:
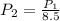
So the volume changes by:
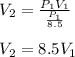
Therefore, it increases by a factor of 8.5.
Regards.