Answer:
71g (assuming experimental data)
Step-by-step explanation:
The balanced equation for this reaction:
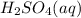 +
+
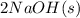 →
→
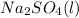 +
+
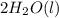
Molar mass of H2SO4 = 98.1 g/mol
molar mass of NaOH = 40g/mol
Molar mass of Na2SO4 = 142.04g/mol
⇒ 1 mole or 98.1g of H2SO4 will yield 1 ole of NaSO4; alternately, 2 moles or 49 ×2 = 80g of NaOH produces 1 mole of NaSO4.
Therefore, limiting reactant is NaOH.
Assuming actual experiment is 20g of NaOH,
1 mole - 40g
x moles - 20g =
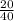 = 0.5 moles
= 0.5 moles
⇒1 mole of Na2SO4 - 142.04g
∴ 0.5 moles = 142.04 × 0.5
= 71.02g