Answer: The actual value of
 is -618 J/mol
is -618 J/mol
Step-by-step explanation:
Relation of ree energy change and equilibrium constant
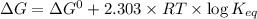
where,
 = Free energy change
= Free energy change
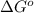 = standard free energy change = +29 kJ/mol =
= standard free energy change = +29 kJ/mol =
R = universal gas constant
T = temperature =
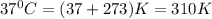
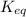 = equilibrium constant =
= equilibrium constant =
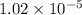
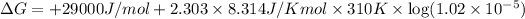
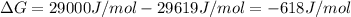
The actual value of
 is -618 J/mol
is -618 J/mol