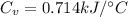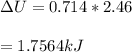Answer:
A constant volume calorimeter (bomb calorimeter) was calibrated by performing in it a reaction in which 5.23 kJ of heat energy was released, causing the calorimeter to rise by 7.33 °C. What is the heat capacity Cy of the calorimeter
Step-by-step explanation:
the heat capacity of the calorimeter is
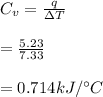
Now that our heat capacity of the calorimeter is 0.714kJ/°C
we can easily calculate the change in internal energy ΔU of the neutralization reaction
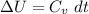
or
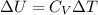
Δ T = 2.46 °C