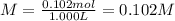Answer:
0.102 M
Step-by-step explanation:
Given data
- Mass of sucrose (solute): 35.0 g
- Volume of solution: 1.000 L
Step 1: Calculate the moles of solute
The molar mass of sucrose is 342.3 g/mol. The moles corresponding to 35.0 g of sucrose are:
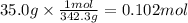
Step 2: Calculate the molarity of sucrose in the solution
The molarity is equal to the moles of solute divided by the liters of solution.