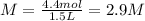Answer:
2.9 M
Step-by-step explanation:
Step 1: Given data
Moles of barium chloride (solute): 4.4 moles
Volume of solution: 1.5 liters
Step 2: Calculate the molarity of barium chloride in the solution
The molarity is a way to quantitatively express the concentration of a solute in a solution. The molarity is equal to the moles of solute divided by the volume, in liters, of solution.