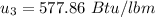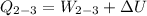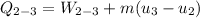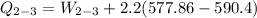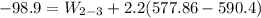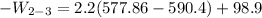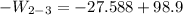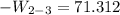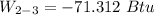Answer:
The heat transfer
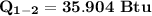
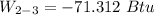
Step-by-step explanation:
At the initial state when P₁ = 10 lbf/in :
We obtain the internal energy u₁ and specific volume v₁.
u₁ =
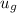 = 574.08 btu/lbm
= 574.08 btu/lbm
v₁ =
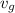 = 2.4746 ft³/lbm
= 2.4746 ft³/lbm
Process 1–2 occurs at constant volume until the temperature is 100°F.
i.e T₂ = 100⁰ F
At T₂ = 100⁰ F : v₁ = v₂ = 2.4746 ft³/lbm
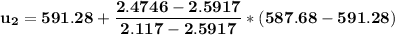
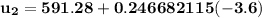
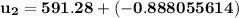
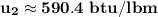
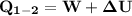
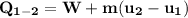
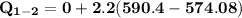
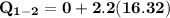
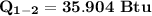
b) the work for Process 2–3, in Btu.
At
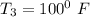 ;
;