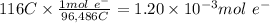Answer:
1.20 × 10⁻³ mol e⁻
Step-by-step explanation:
There is some info missing. I think this is the original question.
Suppose a current of 0.880 A flows through a copper wire for 132 seconds. Calculate how many moles of electrons travel through the wire. Be sure your answer has the correct unit symbol and round your answer to significant digits.
Step 1: Given data
- Intensity of the current (I): 0.880 A (= 0.880 C/s)
Step 2: Calculate the charge, in Coulomb, that travel through the wire
We will find the circulating charge (q) using the following expression.
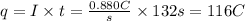
Step 3: Calculate the moles of electrons with a charge of 116 C
We will use the relationship 1 mole of electrons = 96,486 C (Faraday's constant)