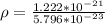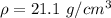Complete Question
The complete question is shown on the first uploaded image
Answer:
The density is
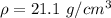
Step-by-step explanation:
From the question we are told that
The lattice constant is
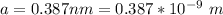
Generally the volume of the unit cell is
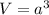
=>
![V = [0.387 *10^(-9)]^2](https://img.qammunity.org/2021/formulas/chemistry/college/n0r95c2s79iyac0rn08w216rw53vbfdvsa.png)
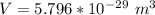
Converting to
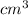 We have
We have
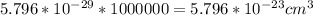
The molar mass of Tungsten is constant with a value
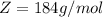
One mole of Tungsten contains
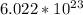 unit cells
unit cells
Where
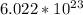 is a constant for the number of atom in one mole of a substance(Tungsten) which is known as Avogadro's constant
is a constant for the number of atom in one mole of a substance(Tungsten) which is known as Avogadro's constant
Now for FCC distance the number of atom per unit cell is n = 4
Mass of Tungsten (M) =
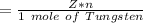
=> Mass of Tungsten (M) =
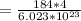
=> Mass of Tungsten (M) =

Now
The density of Tungsten is
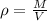
substituting values