Answer:
The standard potential at 25ºC is 3.17 V.
Step-by-step explanation:
The anode in a galvanic cell is the electrode at which oxidation occurs and the cathode is the electrode at which reduction occurs.
The overall cell reaction will be the sum of two half-cell reactions. The standard reduction potentials are:
Mg²⁺ (1.0 M) + 2e⁻ → Mg (s) Eº = -2.37
Ag⁺ (1.0 M) + e⁻ → Ag (s) Eº= +0.80
Since the reactants are in their standard states (1.0 M) and at 25ºC we can write the half-cell reactions as follows:
Anode (oxidation): Mg (s) → Mg²⁺ (1.0 M) + 2e⁻
Cathode (reduction): 2Ag⁺ (1.0 M) + 2e⁻ → 2Ag (s)
Overall: Mg (s) +2 Ag⁺ (1.0 M) + 2e⁻ → 2Ag (s) + Mg²⁺ (1.0 M) + 2e⁻
In order to balance the overall equation we multiply the reduction of Ag⁺ by 2. We can do so because, as an intensive property, E° is not affected by this procedure.
The standard emf of the cell, E°cell , which is composed of a contribution from the anode and a contribution from the cathode, is given by:
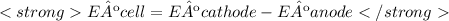
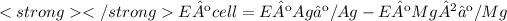
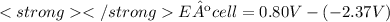
Eº cell = 3.17 V