Answer: -100 kJ
Step-by-step explanation:
Heat of reaction or enthalpy of the reaction is the energy released or absorbed during the course of the reaction.
Heat of reaction is represented by the symbol
 .
.
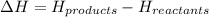
 = enthalpy of reaction = ?
= enthalpy of reaction = ?
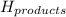 = enthalpy of products = 0 kJ
= enthalpy of products = 0 kJ
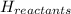 = enthalpy of reactants = 100 kJ
= enthalpy of reactants = 100 kJ
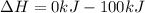

Thus the ΔH for the reaction is -100kJ