Answer: The concentration of
 ions in the solution is 0.0063 M
ions in the solution is 0.0063 M
Step-by-step explanation:
pH or pOH is the measure of acidity or alkalinity of a solution.
pOH is calculated by taking negative logarithm of hydroxide ion concentration.
![pOH=-\log [OH^-]](https://img.qammunity.org/2021/formulas/chemistry/college/hdm1ob4dj6mx2sy3kobrrj91lzbh3927bk.png)
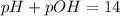
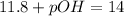
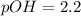
Putting in the values:
![2.2=-\log[OH^-]](https://img.qammunity.org/2021/formulas/chemistry/high-school/q9gt7etqnwx66zjyq3f70i1xwsw86uhdxz.png)
![[OH^-]=](https://img.qammunity.org/2021/formulas/chemistry/high-school/ndpfqq8i6urbrd9v2om0f7es5ryb9fhqf3.png) 0.0063 M
0.0063 M
Thus the concentration of
 ions in the solution is 0.0063 M
ions in the solution is 0.0063 M