Answer:
The amount of ampicillin stock is
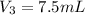
The amount of deionized is 22.5 \ mL
Step-by-step explanation:
From the question we are told that
The volume of stock solution of ampicillin in de-ionized water is
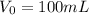
The concentration (in mg ) of ampicillin in de-ionized is
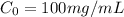
The volume of ampicillin in deionized water to produce is
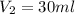
The concentration (in mg ) of ampicillin in de-ionized water to produce is
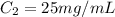
The chemical process can be mathematically represented as
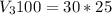
=>
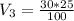
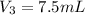
The amount of ampicillin stock is
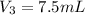 and amount of 25 mL (30 - 7.5 = 22.5 mL ) of deionized water
and amount of 25 mL (30 - 7.5 = 22.5 mL ) of deionized water