Answer:
Mass of other chemicals to be evaporated is 24 grams.
Explanation:
The initial mass of the hand sanitizer = 400 grams, and has an alcohol content of 24%.
mass of its alcohol content =
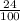 × 400
× 400
= 96 grams
mass of non-alcoholic content = 400 - 96
= 304 grams
If the percentage of alcohol is increased to 30%, then;
mass of its alcohol content =
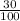 × 400
× 400
= 120 grams
The mass of other chemicals to be evaporated = 120 - 90
= 24 grams